bromine electron configuration|Bromine electron configuration : Clark The bromine electron configuration, denoted as [ Ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. Answers for light downy substance(5 crossword clue, 5 letters. Search for crossword clues found in the Daily Celebrity, NY Times, Daily Mirror, Telegraph and major publications. Find clues for light downy substance(5 or most any crossword answer or clues for crossword answers.
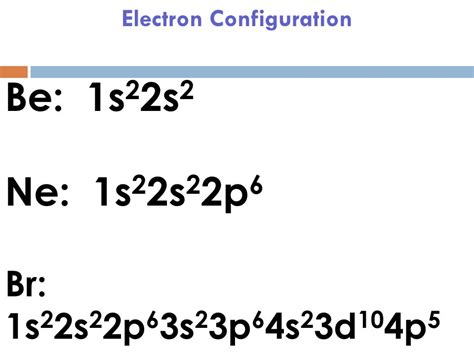
bromine electron configuration,Mar 23, 2023 The bromine electron configuration, denoted as [ Ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. Find the electron configuration of bromine using the aufbau principle and a chart of subshells. See two answers with explanations and links to more information. The properties of chlorine are intermediate between those of iodine and chlorine. Bromine Orbital Diagram. In this article today we are going to tell you about the electron configuration of Bromine, its orbital .Bromine is a deep-red, oily liquid with a sharp smell and a toxicity. Its electron configuration is [Ar] 3d 10 4s 2 4p 5. Learn more about its discovery, biological role, natural .Bromine is a volatile red-brown liquid with the symbol Br and atomic number 35. It belongs to group 17 of the periodic table and has the electron configuration [Ar] 3d 10 4s 2 4p 5. Learn more about its history, .Bromine. Full electron configuration of bromine: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. selenium ← bromine → krypton. Bromine, complete electron configuration.Bromine is a halogen with the symbol Br and atomic number 35. Its electron configuration is [Ar] 3d 10 4s 2 4p 5. Learn more about its history, physical and chemical properties, applications and isotopes.Bromine is a nonmetal with atomic number 35 and chemical symbol Br. Its electron configuration is [Ar] 3d 10 4s 2 4p 5, with valence electrons of 7 and valency electrons of . Bromine unabbreviated electron configuration. Br: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. When there is no noble gas configuration for the starting electrons, this is referred to as an unabbreviated electronic configuration. ground state Bromine electron configuration. The ground state electronic configuration of Br will be 1s 2 2s .
Bromine’s electron configuration is [Ar] 3d10 4s2 4p5. Bromine has 7 valence electrons, rendering it a highly electronegative, reactive element, and prone to ionic reactions. On the Pauling Scale, bromine’s . Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure.The chemical symbol for Bromine is Br. Electron Configuration and Oxidation States of Bromine. Electron configuration of Bromine is [Ar] 3d10 4s2 4p5. Possible oxidation states are +1,3,5/-1. Electron .
What is the electron configuration for bromine? Chemistry Electron Configuration Electron Configuration. 1 Answer Zach Dec 24, 2015 [Ar] #4s^(2)3d^(10)4p^5# Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full .bromine electron configurationBromine. Full electron configuration of bromine: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 selenium ← bromine → krypton. © 2009-2017 | www.prvky.com | kontaktkontakt

The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum .
bromine electron configuration Bromine electron configuration The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum .Electronic configuration of the Bromine atom. Valence electrons. Orbital diagram. Bromine electron configuration. ← Electronic configurations of elements . Br (Bromine) is an element with position number 35 in the periodic table. Located in the IV period. Melting point: -7.3 ℃.Complete ground state electronic configuration for the Bromine atom, Unabbreviated electronic configuration. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. Electrons are filled in atomic orbitals as per the order determined by the Aufbau principle, Pauli Exclusion Principle and Hund’s Rule. As per the Aufbau principle the electrons will occupy the .
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order .

This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .
Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electron configuration of ground state gaseous neutral bromine is [ Ar ]. 3d 10 . 4s 2 . 4p 5 and the term symbol is 2 P 3/2 .Bromine electron configuration Quantum numbers. There are four quantum numbers n, l, m l, and m s.The principal quantum number n is a positive integer (1,2,3,4) and it represents the energy of the orbital.The angular momentum quantum number l, is from 0 to n – 1. The l values of 0, 1, 2, and 3 correspond to the s, p, d and f orbitals, respectively. The magnetic quantum .
This structure is called an electron configuration and is unique to hydrogen. Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells contain one s orbital which can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”).Get the facts about element Bromine (Br) [35] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including decay trees) as well as some historic information. What is bromine's electron configuration? Chemistry Electron Configuration Electron Configuration. 1 Answer BRIAN M. Jul 2, 2016 #Br = 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5# #Br = [Ar] 4s^2 3d^10 4p^5# Explanation: Bromine is .The Electron configuration of bromine is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. Bromine, also known as liquid fire, is defined as a chemical element that is part of the periodic table of elements.
bromine electron configuration|Bromine electron configuration
PH0 · Electronic configuration of bromine ?
PH1 · Electron Configuration Chart of All Elements (Full Chart)
PH2 · Complete Electron Configuration for Bromine (Br, Br
PH3 · Bromine, electron configuration
PH4 · Bromine electron configuration
PH5 · Bromine Electron Configuration (Br) with Orbital Diagram
PH6 · Bromine (Br)
PH7 · Bromine